Knowledge and Skills Statement
The further explanation is designed to be a resource for educators that helps them better understand the topic their students are learning. Further explanations may be written at a more complex level than would be expected for students at the grade level.
Mass
In everyday use, mass and weight are sometimes incorrectly used interchangeably. Students in grade 5 are expected to measure mass, however they are not expected to understand the role gravity plays in differentiating between mass and weight. In science, mass is always measured in metric units (kilograms). Experiences in science with scales and balances will help support students' development of understanding mass versus weight, while in mathematics students will understand units of measurement as customary and metric.
As an educator, it is important to understand the differences between mass and weight.
Weight is measured on a scale and accounts for the downward force of gravity. When measuring mass, an object is placed on one side of a balance and counterweights are placed on the opposite side of the balance. This use of a counterbalance means that the measurement taken is consistent regardless of the force of gravity in an environment. In other words, while the weight of an object measured on a scale is lighter on the Moon than it is on Earth, the mass of the object measured with a balance would be the same.
Physical state and volume
The physical state of matter is partially dependent on the movement of particles in the substance. The faster the particles move, the hotter the solid, liquid, or gas. The slower the particles move, the colder the solid, liquid, or gas. Increasing thermal energy will make particles move faster, causing the substance to heat up. Decreasing thermal energy will make particles slow down, causing the substance to cool.
The physical state, volume, and density of a substance are connected. For example, as its particles move slower and a substance cools, the particles of a gas condense and become closer together. The volume of the substance decreases, and the gas becomes a liquid. The same mass in a smaller volume has a higher density.
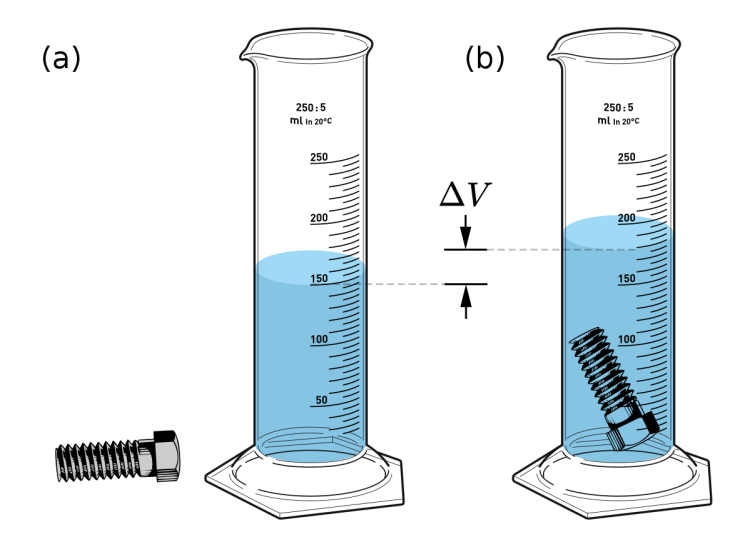
The water displacement method for measuring the volume of a solid involves submerging an object in water and measuring the increase in the water level. If the object floats, gently push it below the water line to take the measurement. In the image below, the volume of the water in the first graduated cylinder is 150 mL. When the bolt was added to the graduated cylinder, the total volume increased to 200 mL. Subtracting the volume of the water (150 mL) from the volume of the water and the bolt (200 mL) provides the volume of the bolt (50 mL). When comparing the volume of a cube to the volume of an irregularly shaped object, it may help some students to understand that one milliliter is equivalent to one centimeter cubed. While the vast majority of substances are more dense as a solid than as a liquid, there are some exceptions. Students may be familiar with ice floating on water. This occurs because of the unique way the particles of water interact and expand as they freeze to form solid ice.
Research
Bobrowsky, Matthew. “SCIENCE 101 Q:What’s a Fun Activity That Combines Science With Art? in Engaging Families. Science and Children 57, no. 9 (July/August 2020):70–73. https://www.jstor.org/stable/27045280.
Summary: This article defines the terms “solubility” and “solvent.” Teachers can help students explore solubility with experiments using everyday items like candy, celery, and coffee filters. Students can add water to M&Ms and watch as the color spreads out into the water because water is a solvent. Similarly, students can use a variety of black markers on a coffee filter placed in water. Students will observe the different colors contained in black markers spread out into the solvent. These colors will disperse at different speeds to the different sizes of molecules in each color. Similar activities can be done with celery and food color to show students how different colors move in various solvents.
Research
Vincent, Dan, Darlinda Cassel, and Jeanie Milligan. “Will It Float?” Science and Children 45, no. 6 (February 2008): 36–39. https://www.proquest.com/docview/236960208?pq-origsite=gscholar&fromopenview=true&sourcetype=Scholarly%20Journals.
Summary: This experiment was inspired by upper-elementary students’ responses to a previous activity where they found that sometimes larger objects can weigh less than smaller objects. This lesson is based on the 5E model and is used to help students explore mass, density, and volume. Students predicted whether different items would sink or float and were confused by why some types of wood and rocks would float while many others would sink. Students also predicted the mass of objects of different sizes and recorded their observations. Students tested whether objects made of plastic and aluminum would float and measured their volume and mass along with measuring the mass of the water. They then graphed this data using different colors to explain the relation between mass, volume, and density. Teachers evaluated student understanding throughout the activity by observing conversations among students and asking questions.