Knowledge and Skills Statement
The further explanation is designed to be a resource for educators that helps them better understand the topic their students are learning. Further explanations may be written at a more complex level than would be expected for students at the grade level.
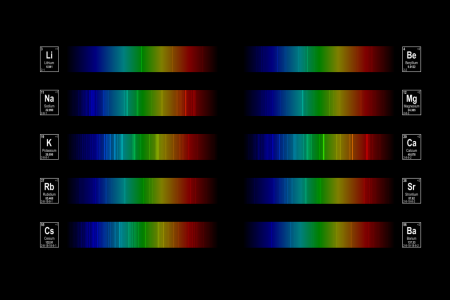
The bright lines in the diagram are the emission lines for each element, shown against a background of the visible electromagnetic spectrum.
BélaBéla, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
Research
Körhasan, Nilüfer Didiş, and Lu Wang. "Students’ Mental Models of Atomic Spectra." Chemistry Education Research and Practice 17, no. 4 (2016):743–755. https://doi.org/10.1039/c6rp00051g
Summary: Mental modeling, which is a theory about knowledge organization, has been recently studied by science educators to examine students' understanding of scientific concepts. This qualitative study investigates undergraduate students' mental models of atomic spectra.