Learning Objectives
Learning Objectives
In this section, you will explore the following questions:
- What are functions of proteins in cells and tissues?
- What is the relationship between amino acids and proteins?
- What are the four levels of protein organization?
- What is the relationship between protein shape and function?
Connection for AP® Courses
Connection for AP® Courses
Proteins are long chains of different sequences of the 20 amino acids that each contain an amino group (-NH2), a carboxyl group (-COOH), and a variable group. Think of how many protein words can be made with 20 amino acid letters. Each amino acid is linked to its neighbor by a peptide bond formed by a dehydration reaction. A long chain of amino acids is known as a polypeptide. Proteins serve many functions in cells. They act as enzymes that catalyze chemical reactions, provide structural support, regulate the passage of substances across the cell membrane, protect against disease, and coordinate cell signaling pathways. Protein structure is organized at four levels: primary, secondary, tertiary, and quaternary. The primary structure is the unique sequence of amino acids. A change in just one amino acid can change protein structure and function. For example, sickle cell anemia results from just one amino acid substitution in a hemoglobin molecule consisting of 574 amino acids. The secondary structure consists of the local folding of the polypeptide by hydrogen bond formation; leading to the α helix and β pleated sheet conformations. In the tertiary structure, various interactions, e.g., hydrogen bonds, ionic bonds, disulfide linkages, and hydrophobic interactions between R groups, contribute to the folding of the polypeptide into different three-dimensional configurations. Most enzymes are of tertiary configuration. If a protein is denatured, loses its three-dimensional shape, it may no longer be functional. Environmental conditions such as temperature and pH can denature proteins. Some proteins, such as hemoglobin, are formed from several polypeptides, and the interactions of these subunits form the quaternary structure of proteins.
Information presented and the examples highlighted in the section, support concepts and Learning Objectives outlined in Big Idea 4 of the AP® Biology Curriculum Framework. The Learning Objectives listed in the Curriculum Framework provide a transparent foundation for the AP® Biology course, an inquiry-based laboratory experience, instructional activities, and AP® exam questions. A Learning Objective merges required content with one or more of the seven science practices.
| Big Idea 4 | Biological systems interact, and these systems and their interactions possess complex properties. |
| Enduring Understanding 4.A | Interactions within biological systems lead to complex properties. |
| Essential Knowledge | 4.A.1 The subcomponents of biological molecules and their sequence determine the properties of that molecule. |
| Science Practice | 7.1 The student can connect phenomena and models across spatial and temporal scales. |
| Learning Objective | 4.1 The student is able to explain the connection between the sequence and the subcomponents of a biological polymer and its properties. |
| Science Practice | 1.3 The student can refine representations and models of natural or man-made phenomena and systems in the domain. |
| Learning Objective | 4.2 The student is able to refine representations and models to explain how the subcomponents of a biological polymer and their sequence determine the properties of that polymer. |
| Science Practice | 6.1 The student can justify claims with evidence. |
| Science Practice | 6.4 The student can make claims and predictions about natural phenomena based on scientific theories and models. |
| Learning Objective | 4.3 The student is able to use models to predict and justify that changes in the subcomponents of a biological polymer affect the functionality of the molecules. |
The Science Practices Assessment Ancillary contains additional test questions for this section that will help you prepare for the AP® exam. These questions address the following standards:
- [APLO 1.14]
- [APLO 2.12]
- [APLO 4.1]
- [APLO 4.3]
- [APLO 4.15]
- [APLO 4.22]
Types and Functions of Proteins
Types and Functions of Proteins
Proteins are one of the most abundant organic molecules in living systems and have the most diverse range of functions of all macromolecules. Proteins may be structural, regulatory, contractile, or protective; they may serve in transport, storage, or membranes; or, they may be toxins or enzymes. Each cell in a living system may contain thousands of proteins, each with a unique function. Their structures, like their functions, vary greatly. They are all, however, polymers of amino acids, arranged in a linear sequence.
Enzymes, which are produced by living cells, are catalysts in biochemical reactions, like digestion, and are usually complex or conjugated proteins. Each enzyme is specific for the substrate—a reactant that binds to an enzyme—it acts on. The enzyme may help in breakdown, rearrangement, or synthesis reactions. Enzymes that break down their substrates are called catabolic enzymes; enzymes that build more complex molecules from their substrates are called anabolic enzymes, and enzymes that affect the rate of reaction are called catalytic enzymes. It should be noted that all enzymes increase the rate of reaction and, therefore, are considered to be organic catalysts. An example of an enzyme is salivary amylase, which hydrolyzes its substrate amylose, a component of starch.
Hormones are chemical-signaling molecules, usually small proteins or steroids, secreted by endocrine cells that act to control or regulate specific physiological processes, including growth, development, metabolism, and reproduction. For example, insulin is a protein hormone that helps to regulate the blood glucose level. The primary types and functions of proteins are listed in Table 3.1.
| Protein Types and Functions | ||
|---|---|---|
| Type | Examples | Functions |
| Digestive Enzymes | Amylase, lipase, pepsin, trypsin | Help in digestion of food by catabolizing nutrients into monomeric units |
| Transport | Hemoglobin, albumin | Carry substances in the blood or lymph throughout the body |
| Structural | Actin, tubulin, keratin | Construct different structures, like the cytoskeleton |
| Hormones | Insulin, thyroxine | Coordinate the activity of different body systems |
| Defense | Immunoglobulins | Protect the body from foreign pathogens |
| Contractile | Actin, myosin | Effect muscle contraction |
| Storage | Legume storage proteins, egg white (albumin) | Provide nourishment in early development of the embryo and the seedling |
Proteins have different shapes and molecular weights; some proteins are globular in shape, whereas others are fibrous in nature. For example, hemoglobin is a globular protein, but collagen, found in our skin, is a fibrous protein. Protein shape is critical to its function, and this shape is maintained by many different types of chemical bonds. Changes in temperature, pH, and exposure to chemicals may lead to permanent changes in the shape of the protein, leading to loss of function, known as denaturation. All proteins are made up of different arrangements of the same 20 types of amino acids.
Amino Acids
Amino Acids
Amino acids are the monomers that make up proteins. Each amino acid has the same fundamental structure, which consists of a central carbon atom, also known as the alpha (α) carbon, bonded to an amino group (NH2), a carboxyl group (COOH), and to a hydrogen atom. Every amino acid also has another atom or group of atoms bonded to the central atom known as the R group (Figure 3.24).
The name amino acid is derived from the fact that they contain both amino group and carboxyl-acid-group in their basic structure. As mentioned, there are 20 amino acids present in proteins. Ten of these are considered essential amino acids in humans because the human body cannot produce them and they are obtained from the diet. For each amino acid, the R group, or side chain, is different (Figure 3.25).
Visual Connection
Which categories of amino acids would you expect to find on the surface of a soluble protein, and which would you expect to find in the interior?
- Polar and charged amino acids will be found on the surface. Nonpolar amino acids will be found in the interior.
- Polar and charged amino acids will be found on the surface. Nonpolar amino acids will be found in the interior.
- Nonpolar and charged amino acids will be on the surface while polar amino acids will be deeper within the protein.
- Nonpolar and uncharged proteins will be found on the surface as well as in the interior.
The chemical nature of the side chain determines the nature of the amino acid—that is, whether it is acidic, basic, polar, or nonpolar. For example, the amino acid glycine has a hydrogen atom as the R group. Amino acids such as valine, methionine, and alanine are nonpolar or hydrophobic in nature, while amino acids such as serine, threonine, and cysteine are polar and have hydrophilic side chains. The side chains of lysine and arginine are positively charged, and therefore these amino acids are also known as basic amino acids. Proline has an R group that is linked to the amino group, forming a ring-like structure. Proline is an exception to the standard structure of an animo acid since its amino group is not separate from the side chain (Figure 3.25).
Amino acids are represented by a single upper case letter or a three-letter abbreviation. For example, valine is known by the letter V or the three-letter symbol val. Just as some fatty acids are essential to a diet, some amino acids are necessary as well. They are known as essential amino acids, and in humans they include isoleucine, leucine, and cysteine. Essential amino acids refer to those necessary for construction of proteins in the body, although not produced by the body; which amino acids are essential varies from organism to organism.
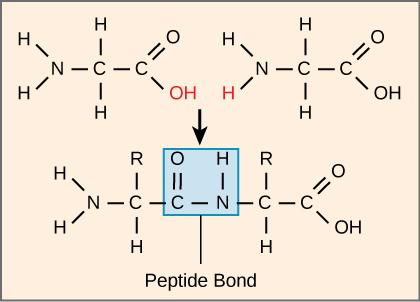
The sequence and the number of amino acids ultimately determine the protein's shape, size, and function. Each amino acid is attached to another amino acid by a covalent bond, known as a peptide bond, which is formed by a dehydration reaction. The carboxyl group of one amino acid and the amino group of the incoming amino acid combine, releasing a molecule of water. The resulting bond is the peptide bond (Figure 3.26).
The products formed by such linkages are called peptides. As more amino acids join to this growing chain, the resulting chain is known as a polypeptide. Each polypeptide has a free amino group at one end. This end is called the N terminal, or the amino terminal, and the other end has a free carboxyl group, also known as the C or carboxyl terminal. While the terms polypeptide and protein are sometimes used interchangeably, a polypeptide is technically a polymer of amino acids, whereas the term protein is used for a polypeptide or polypeptides that have combined together, often have bound non-peptide prosthetic groups, have a distinct shape, and have a unique function. After protein synthesis (translation), most proteins are modified. These are known as post-translational modifications. They may undergo cleavage, phosphorylation, or may require the addition of other chemical groups. Only after these modifications is the protein completely functional.
Link to Learning
Click through the steps of protein synthesis in this interactive tutorial.
Why is the process of protein synthesis critical to life?
- Protein is the body’s preferred source for energy for rapid energy production.
- Protein is stored in the liver and muscles to supply energy for future use.
- Protein is required for tissue formation and constitutes hormones and enzymes.
- Protein is required for the absorption of all fat soluble vitamins.
Evolution Connection
Cytochrome c is an important component of the electron transport chain, a part of cellular respiration, and it is normally found in the cellular organelle, the mitochondrion. This protein has a heme prosthetic group, and the central ion of the heme gets alternately reduced and oxidized during electron transfer. Because this essential protein’s role in producing cellular energy is crucial, it has changed very little over millions of years. Protein sequencing has shown that there is a considerable amount of cytochrome c amino acid sequence homology among different species; in other words, evolutionary kinship can be assessed by measuring the similarities or differences among various species’ DNA or protein sequences.
Scientists have determined that human cytochrome c contains 104 amino acids. For each cytochrome c molecule from different organisms that has been sequenced to date, 37 of these amino acids appear in the same position in all samples of cytochrome c. This indicates that there may have been a common ancestor. On comparing the human and chimpanzee protein sequences, no sequence difference was found. When human and rhesus monkey sequences were compared, the single difference found was in one amino acid. In another comparison, human to yeast sequencing shows a difference in the 44th position.
- Rhesus monkeys are more closely related to humans than chimpanzees.
- Chimpanzees are more closely related to rhesus monkeys than to humans.
- Humans are related to chimpanzees, but are not related to rhesus monkeys.
- Chimpanzees are more closely related to humans than rhesus monkeys.
Protein Structure
Protein Structure
As discussed earlier, the shape of a protein is critical to its function. For example, an enzyme can bind to a specific substrate at a site known as the active site. If this active site is altered because of local changes or changes in overall protein structure, the enzyme may be unable to bind to the substrate. To understand how the protein gets its final shape or conformation, we need to understand the four levels of protein structure: primary, secondary, tertiary, and quaternary.
Primary Structure
The unique sequence of amino acids in a polypeptide chain is its primary structure. For example, the pancreatic hormone insulin has two polypeptide chains, A and B, and they are linked together by disulfide bonds. The N terminal amino acid of the A chain is glycine, whereas the C terminal amino acid is asparagine (Figure 3.27). The sequences of amino acids in the A and B chains are unique to insulin.
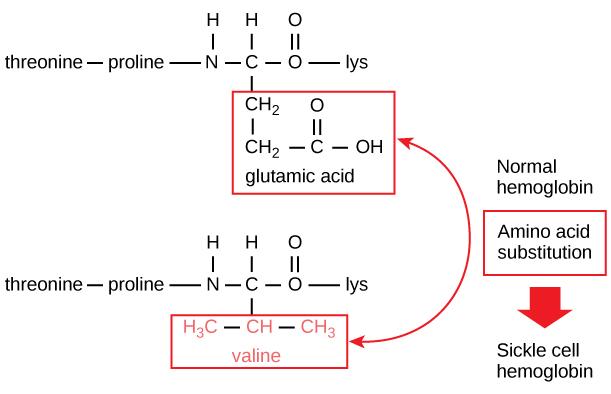
The unique sequence for every protein is ultimately determined by the gene encoding the protein. A change in nucleotide sequence of the gene’s coding region may lead to a different amino acid being added to the growing polypeptide chain, causing a change in protein structure and function. In sickle cell anemia, the hemoglobin β chain—a small portion of which is shown in Figure 3.28—has a single amino acid substitution, causing a change in protein structure and function. Specifically, the amino acid glutamic acid is substituted by valine in the β chain. What is most remarkable to consider is that a hemoglobin molecule is made up of two alpha chains and two beta chains that each consist of about 150 amino acids. The molecule, therefore, has about 600 amino acids. The structural difference between a normal hemoglobin molecule and a sickle cell molecule—which dramatically decreases life expectancy—is a single amino acid of the 600. What is even more remarkable is that those 600 amino acids are encoded by three nucleotides each, and the mutation is caused by a single base change, or point mutation, one in 1800 bases.
Because of this change of one amino acid in the chain, hemoglobin molecules form long fibers that distort the biconcave, or disc-shaped, red blood cells and assume a crescent or sickle shape, which clogs arteries (Figure 3.29). This can lead to myriad serious health problems, such as breathlessness, dizziness, headaches, and abdominal pain for those affected by this disease.
Secondary Structure
The local folding of the polypeptide in some regions gives rise to the secondary structure of the protein. The most common are the α-helix and β-pleated sheet structures (Figure 3.30). Both structures are held together by hydrogen bonds. In the α-helix structure, the hydrogen bonds form between the oxygen atom in the carbonyl group in one amino acid and another amino acid that is four amino acids farther along the chain.
Every helical turn in an alpha helix has 3.6 amino acid residues. The R groups, the variant groups, of the polypeptide protrude out from the α-helix chain. In the β-pleated sheet, the pleats are formed by hydrogen bonding between atoms on the backbone of the polypeptide chain. The R groups are attached to the carbons and extend above and below the folds of the pleat. The pleated segments align parallel or antiparallel to each other, and hydrogen bonds form between the partially positive nitrogen atom in the amino group and the partially negative oxygen atom in the carbonyl group of the peptide backbone. The α-helix and β-pleated sheet structures are found in most globular and fibrous proteins and they play an important structural role.
Tertiary Structure
The unique three-dimensional structure of a polypeptide is its tertiary structure (Figure 3.31). This structure is in part due to chemical interactions at work on the polypeptide chain. Primarily, the interactions among R groups creates the complex three-dimensional tertiary structure of a protein. The nature of the R groups found in the amino acids involved can counteract the formation of the hydrogen bonds described for standard secondary structures. For example, R groups with like charges are repelled by each other and those with unlike charges are attracted to each other—ionic bonds. When protein folding takes place, the hydrophobic R groups of nonpolar amino acids lay in the interior of the protein, whereas the hydrophilic R groups lay on the outside. The former types of interactions are also known as hydrophobic interactions. Interaction between cysteine side chains forms disulfide linkages in the presence of oxygen, the only covalent bond forming during protein folding.
All of these interactions, weak and strong, determine the final three-dimensional shape of the protein. When a protein loses its three-dimensional shape, it may no longer be functional.
Quaternary Structure
In nature, some proteins are formed from several polypeptides, also known as subunits, and the interaction of these subunits forms the quaternary structure. Weak interactions between the subunits help to stabilize the overall structure. For example, insulin—a globular protein—has a combination of hydrogen bonds and disulfide bonds that cause it to be mostly clumped into a ball shape. Insulin starts out as a single polypeptide and loses some internal sequences in the presence of post-translational modification after the formation of the disulfide linkages that hold the remaining chains together. Silk—a fibrous protein—however, has a β-pleated sheet structure that is the result of hydrogen bonding between different chains.
The four levels of protein structure—primary, secondary, tertiary, and quaternary—are illustrated in Figure 3.32.
Denaturation and Protein Folding
Denaturation and Protein Folding
Each protein has its own unique sequence and shape that are held together by chemical interactions. If the protein is subject to changes in temperature, pH, or exposure to chemicals, the protein structure may change, losing its shape without losing its primary sequence in what is known as denaturation. Denaturation is often reversible because the primary structure of the polypeptide is conserved in the process if the denaturing agent is removed, allowing the protein to resume its function. Sometimes denaturation is irreversible, leading to loss of function. One example of irreversible protein denaturation is when an egg is fried. The albumin protein in the liquid egg white is denatured when placed in a hot pan. Not all proteins are denatured at high temperatures; for instance, bacteria that survive in hot springs have proteins that function at temperatures close to boiling. The stomach is also very acidic, has a low pH, and denatures proteins as part of the digestion process; however, the digestive enzymes of the stomach retain their activity under these conditions.
Protein folding is critical to its function. It was originally thought that the proteins themselves were responsible for the folding process. Only recently was it found that often they receive assistance in the folding process from protein helpers known as chaperones, or chaperonins, that associate with the target protein during the folding process. They act by preventing aggregation of polypeptides that make up the complete protein structure, and they disassociate from the protein once the target protein is folded.
Link to Learning
For an additional perspective on proteins, view this animation called Biomolecules: The Proteins.
Vegans are people who do not consume any animal products in their diet. Why do vegans need to pay special attention to the protein that they eat?
- Plant proteins contain all of the essential as well as non-essential amino acids.
- It is more difficult to obtain all essential amino acids from single plant sources.
- Plant proteins contain only non-essential amino acids.
- Plants proteins do not have all of the non-essential amino acids, but do contain the essential amino acids.
Science Practice Connection for AP® Courses
Think About It
- Predict what happens if even one amino acid is substituted for another in a polypeptide and provide a specific example.
- What categories of amino acids would you expect to find on the surface of a soluble protein, and which would you expect to find in the interior? What distribution of amino acids would you expect to find in a protein embedded in a lipid bilayer of a plasma cell membrane?
Activity
Folding is an important property of proteins, especially enzymes. Proteins have a narrow range of conditions in which they fold properly. Outside that range, proteins can unfold, or denature, and often cannot refold and become functional again. Investigate one disease that results from improper folding of a protein. Describe causes of the unfolding and consequences to the molecular structure of the polypeptide that result in the disease.
Disclaimer
This section may include links to websites that contain links to articles on unrelated topics. See the preface for more information.