Learning Objectives
Learning Objectives
In this section, you will explore the following questions:
- What are examples of physical and chemical immune barriers?
- What are the immediate and induced immune responses?
- What are natural killer cells, and what is their role in immunity?
- What are the features of histocompatibility class I molecules?
- How do the proteins in a complement system function to destroy extracellular pathogens?
Connection for AP® Courses
Connection for AP® Courses
Much of the information about the different organ systems of vertebrate animals is not within the scope for AP®. The immune system, however, was chosen for in-depth exploration because all organisms, including humans, must maintain dynamic homeostasis to survive within changing environments. Even the simplest multicellular eukaryotes, like sponges and Cnidarians, have developed cells that specialize in immune defenses to protect against disruptions to homeostasis. News headlines warn us of outbreaks of diseases, including Ebola, measles, flu, and insect-borne viruses such as West Nile and chikungunya, that spread rapidly through populations, often with devastating consequences. We also hear about the emergence of new infections, especially ones caused by bacteria that have evolved resistance to antibiotics.
Immune systems in animals range from a loose cluster of phagocytic cells in sponges to complex interactions of molecules, cells, tissues, and organs that provide immunity in mammals. Components of the immune system constantly search the body for signs of disease-causing microorganisms called pathogens. Immune factors mobilize, identify the nature of the pathogen, strengthen the corresponding cells and molecules to combat the infection, and then halt the immune response after the infection is cleared to avoid unnecessary host cell damage. Because of its programmable memory system, the immune system can remember pathogens and initiate a more rapid response upon re-exposure. The immune response can be either innate or adaptive. The adaptive immune response stores information about past infections and mounts pathogen-specific defense. The innate immune response is always present and defends against all pathogens.
Despite the barriers of skin, tears, and mucus, pathogens may still enter the body. The innate immune system responds with inflammation, pathogen engulfment, and secretion of immune factors and proteins. Several types of cells are involved in the innate immune system, including mast cells that release histamines (causing those annoying symptoms associated with allergies and colds), macrophages that consume pathogens and cancer cells, natural killer (NK) cells that destroy tumor cells and virus-infected cells, several types of white blood cells, and even protective proteins like complement and interferon. We know from experience, however, that these barriers can fail. Fortunately, adaptive immune responses provide another, more specific line of defense.
Information presented and the examples highlighted in the section support concepts outlined in Big Idea 2 of the AP® Biology Curriculum Framework. The AP® Learning Objectives listed in the Curriculum Framework provide a transparent foundation for the AP® Biology course, an inquiry-based laboratory experience, instructional activities, and AP® exam questions. A learning objective merges required content with one or more of the seven science practices.
| Big Idea 2 | Biological systems utilize free energy and molecular building blocks to grow, to reproduce, and to maintain dynamic homeostasis. |
| Enduring Understanding 2.D | Growth and dynamic homeostasis of a biological system are influenced by changes in the system’s environment. |
| Essential Knowledge | 2.D.4 Plants and animals have a variety of chemical defenses against infections that affect dynamic homeostasis. |
| Science Practice | 1.1 The student can create representations and models of natural or man-made phenomena and systems in the domain. |
| Science Practice | 1.2 The student can describe representations and models of natural or man-made phenomena and systems in the domain. |
| Learning Objective | 2.30 The student can create representations or models to describe nonspecific immune defenses in animals. |
The immune system comprises both innate and adaptive immune responses. Innate immunity occurs naturally because of genetic factors or physiology; it is not induced by infection or vaccination but works to reduce the workload for the adaptive immune response. Both the innate and adaptive levels of the immune response involve secreted proteins, receptor-mediated signaling, and intricate cell-to-cell communication. The innate immune system developed early in animal evolution, roughly a billion years ago, as an essential response to infection. Innate immunity has a limited number of specific targets: any pathogenic threat triggers a consistent sequence of events that can identify the type of pathogen and either clear the infection independently or mobilize a highly specialized adaptive immune response. For example, tears and mucus secretions contain microbicidal factors.
Physical and Chemical Barriers
Physical and Chemical Barriers
Before any immune factors are triggered, the skin functions as a continuous, impassable barrier to potentially infectious pathogens. Pathogens are killed or inactivated on the skin by desiccation (drying out) and by the skin’s acidity. In addition, beneficial microorganisms that coexist on the skin compete with invading pathogens, preventing infection. Regions of the body that are not protected by skin (such as the eyes and mucus membranes) have alternative methods of defense, such as tears and mucus secretions that trap and rinse away pathogens, and cilia in the nasal passages and respiratory tract that push the mucus with the pathogens out of the body. Throughout the body are other defenses, such as the low pH of the stomach (which inhibits the growth of pathogens), blood proteins that bind and disrupt bacterial cell membranes, and the process of urination (which flushes pathogens from the urinary tract).
Despite these barriers, pathogens may enter the body through skin abrasions or punctures, or by collecting on mucosal surfaces in large numbers that overcome the mucus or cilia. Some pathogens have evolved specific mechanisms that allow them to overcome physical and chemical barriers. When pathogens do enter the body, the innate immune system responds with inflammation, pathogen engulfment, and secretion of immune factors and proteins.
Pathogen Recognition
Pathogen Recognition
An infection may be intracellular or extracellular, depending on the pathogen. All viruses infect cells and replicate within those cells (intracellularly), whereas bacteria and other parasites may replicate intracellularly or extracellularly, depending on the species. The innate immune system must respond accordingly: by identifying the extracellular pathogen and/or by identifying host cells that have already been infected. When a pathogen enters the body, cells in the blood and lymph detect the specific pathogen-associated molecular patterns (PAMPs) on the pathogen’s surface. PAMPs are carbohydrate, polypeptide, and nucleic acid signatures that are expressed by viruses, bacteria, and parasites but which differ from molecules on host cells. The immune system has specific cells, described in Figure 33.2 and shown in Figure 33.3, with receptors that recognize these PAMPs. A macrophage is a large phagocytic cell that engulfs foreign particles and pathogens. Macrophages recognize PAMPs via complementary pattern recognition receptors (PRRs). PRRs are molecules on macrophages and dendritic cells that are in contact with the external environment. A monocyte is a type of white blood cell that circulates in the blood and lymph and differentiates into macrophages after it moves into infected tissue. Dendritic cells bind molecular signatures of pathogens and promote pathogen engulfment and destruction. Toll-like receptors (TLRs) are a type of PRR that recognizes molecules that are shared by pathogens but distinguishable from host molecules. TLRs are present in invertebrates as well as vertebrates and appear to be one of the most ancient components of the immune system. TLRs have also been identified in the mammalian nervous system.
Effects of Cytokine Release
The binding of PRRs with PAMPs triggers the release of cytokines, which signal that a pathogen is present and needs to be destroyed along with any infected cells. A cytokine is a chemical messenger that regulates cell differentiation (form and function), proliferation (production), and gene expression to affect immune responses. At least 40 types of cytokines exist in humans that differ in terms of the cell type that produces them, the cell type that responds to them, and the changes they produce. One type of cytokine, interferon, is illustrated in Figure 33.4.
One subclass of cytokines is the interleukin (IL), so named because they mediate interactions between leukocytes (white blood cells). Interleukins are involved in bridging the innate and adaptive immune responses. In addition to being released from cells after PAMP recognition, cytokines are released by the infected cells which bind to nearby uninfected cells and induce those cells to release cytokines, which results in a cytokine burst.
A second class of early-acting cytokines are interferons, which are released by infected cells as warnings to nearby uninfected cells. One of the functions of an interferon is to inhibit viral replication. They also have other important functions, such as tumor surveillance. Interferons work by signaling neighboring uninfected cells to destroy RNA and reduce protein synthesis, signaling neighboring infected cells to undergo apoptosis (programmed cell death), and activating immune cells.
In response to interferons, uninfected cells alter their gene expression, which increases the cells’ resistance to infection. One effect of interferon-induced gene expression is sharply reduced cellular protein synthesis. Virally infected cells produce more viruses by synthesizing large quantities of viral proteins. Thus, by reducing protein synthesis, a cell becomes resistant to viral infection.
Phagocytosis and Inflammation
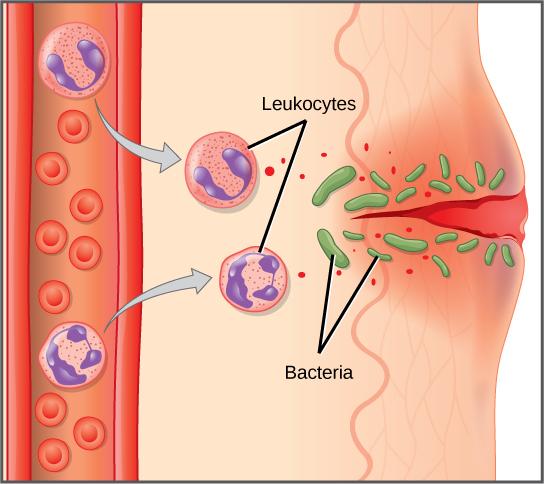
The first cytokines to be produced are pro-inflammatory; that is, they encourage inflammation, the localized redness, swelling, heat, and pain that result from the movement of leukocytes and fluid through increasingly permeable capillaries to a site of infection. The population of leukocytes that arrives at an infection site depends on the nature of the infecting pathogen. Both macrophages and dendritic cells engulf pathogens and cellular debris through phagocytosis. A neutrophil is also a phagocytic leukocyte that engulfs and digests pathogens. Neutrophils, shown in Figure 33.3, are the most abundant leukocytes of the immune system. Neutrophils have a nucleus with two to five lobes, and they contain organelles, called lysosomes, that digest engulfed pathogens. An eosinophil is a leukocyte that works with other eosinophils to surround a parasite; it is involved in the allergic response and in protection against helminthes (parasitic worms).
Neutrophils and eosinophils are particularly important leukocytes that engulf large pathogens, such as bacteria and fungi. A mast cell is a leukocyte that produces inflammatory molecules, such as histamine, in response to large pathogens. A basophil is a leukocyte that, like a neutrophil, releases chemicals to stimulate the inflammatory response as illustrated in Figure 33.5. Basophils are also involved in allergy and hypersensitivity responses and induce specific types of inflammatory responses. Eosinophils and basophils produce additional inflammatory mediators to recruit more leukocytes. A hypersensitive immune response to harmless antigens, such as in pollen, often involves the release of histamine by basophils and mast cells.
Cytokines also send feedback to cells of the nervous system to bring about the overall symptoms of feeling sick, which include lethargy, muscle pain, and nausea. These effects may have evolved because the symptoms encourage the individual to rest and prevent them from spreading the infection to others. Cytokines also increase the core body temperature, resulting in a fever, which causes the liver to withhold iron from the blood. Without iron, certain pathogens, such as some bacteria, are unable to replicate; this is called nutritional immunity.
Link to Learning
Watch this 23-second stop-motion video showing a neutrophil that searches for and engulfs fungus spores during an elapsed time of about 79 minutes.
- Neutrophils phagocytize pathogens invading the body and release chemical histamines that cause pathogen destruction and removal from the body. This prevents pathogens from producing toxic compounds that harm cells.
- Neutrophils phagocytize pathogens invading the body, resulting in their death and removal from the body. This prevents pathogens from multiplying or producing toxic compounds that harm human cells.
- Neutrophils are phagocytic and are the first responders to infection. They produce large quantities of cytokines, which cause pathogen destruction and removal from the body.
- Neutrophils produce cytokines that help phagocytes to recognize foreign material that will destroy and remove pathogens from the body.
Natural Killer Cells
Natural Killer Cells
Lymphocytes are leukocytes that are histologically identifiable by their large, darkly staining nuclei; they are small cells with very little cytoplasm, as shown in Figure 33.6. Infected cells are identified and destroyed by natural killer (NK) cells, lymphocytes that can kill cells infected with viruses or tumor cells (abnormal cells that uncontrollably divide and invade other tissue). T cells and B cells of the adaptive immune system are also classified as lymphocytes. T cells are lymphocytes that mature in the thymus gland, and B cells are lymphocytes that mature in the bone marrow. NK cells identify intracellular infections, especially from viruses, by the altered expression of major histocompatibility class (MHC) I molecules on the surface of infected cells. MHC I molecules are proteins on the surfaces of all nucleated cells, thus they are scarce on red blood cells and platelets which are non-nucleated. The function of MHC I molecules is to display fragments of proteins from the infectious agents within the cell to T-cells; healthy cells will be ignored, while non-self or foreign proteins will be attacked by the immune system. MHC II molecules are found mainly on cells containing antigens (non-self proteins) and on lymphocytes. MHC II molecules interact with helper T-cells to trigger the appropriate immune response, which may include the inflammatory response.
An infected cell (or a tumor cell) is usually incapable of synthesizing and displaying MHC I molecules appropriately. The metabolic resources of cells infected by some viruses produce proteins that interfere with MHC I processing and/or trafficking to the cell surface. The reduced MHC I on host cells varies from virus to virus and results from active inhibitors being produced by the viruses. This process can deplete host MHC I molecules on the cell surface, which NK cells detect as unhealthy or abnormal while searching for cellular MHC I molecules. Similarly, the dramatically altered gene expression of tumor cells leads to expression of extremely deformed or absent MHC I molecules that also signal unhealthy or abnormal.
NK cells are always active; an interaction with normal, intact MHC I molecules on a healthy cell disables the killing sequence, and the NK cell moves on. After the NK cell detects an infected or tumor cell, its cytoplasm secretes granules comprised of perforin, a destructive protein that creates a pore in the target cell. Granzymes are released along with the perforin in the immunological synapse. A granzyme is a protease that digests cellular proteins and induces the target cell to undergo programmed cell death, or apoptosis. Phagocytic cells then digest the cell debris left behind. NK cells are constantly patrolling the body and are an effective mechanism for controlling potential infections and preventing cancer progression.
Complement
Complement
An array of approximately 20 types of soluble proteins, called a complement system, functions to destroy extracellular pathogens. Cells of the liver and macrophages synthesize complement proteins continuously; these proteins are abundant in the blood serum and are capable of responding immediately to infecting microorganisms. The complement system is so named because it is complementary to the antibody response of the adaptive immune system. Complement proteins bind to the surfaces of microorganisms and are particularly attracted to pathogens that are already bound by antibodies. Binding of complement proteins occurs in a specific and highly regulated sequence, with each successive protein being activated by cleavage and/or structural changes induced upon binding of the preceding protein(s). After the first few complement proteins bind, a cascade of sequential binding events follows in which the pathogen rapidly becomes coated in complement proteins.
Complement proteins perform several functions. The proteins serve as a marker to indicate the presence of a pathogen to phagocytic cells, such as macrophages and B cells, and enhance engulfment; this process is called opsonization. Certain complement proteins can combine to form attack complexes that open pores in microbial cell membranes. These structures destroy pathogens by causing their contents to leak, as illustrated in Figure 33.7.
Disclaimer
This section may include links to websites that contain links to articles on unrelated topics. See the preface for more information.