Learning Objectives
Learning Objectives
In this section, you will explore the following questions:
- What is the role of carbohydrates in cells and in the extracellular materials of animals and plants?
- What are the different classifications of carbohydrates?
- How are monosaccharide building blocks assembled into disaccharides and complex polysaccharides?
Connection for AP® Courses
Connection for AP® Courses
Carbohydrates provide energy for the cell and structural support to plants, fungi, and arthropods such as insects, spiders, and crustaceans. Consisting of carbon, hydrogen, and oxygen in the ratio CH2O or carbon hydrated with water, carbohydrates are classified as monosaccharides, disaccharides, and polysaccharides depending on the number of monomers in the macromolecule. Monosaccharides are linked by glycosidic bonds that form as a result of dehydration synthesis. Glucose, galactose, and fructose are common isomeric monosaccharides, whereas sucrose or table sugar is a disaccharide. Examples of polysaccharides include cellulose and starch in plants and glycogen in animals. Although storing glucose in the form of polymers like starch or glycogen makes it less accessible for metabolism, this prevents it from leaking out of cells or creating a high osmotic pressure that could cause excessive water uptake by the cell. Insects have a hard outer skeleton made of chitin, a unique nitrogen-containing polysaccharide.
Information presented and the examples highlighted in the section support concepts and Learning Objectives outlined in Big Idea 4 of the AP® Biology Curriculum Framework. The Learning Objectives listed in the Curriculum Framework provide a transparent foundation for the AP® Biology course, an inquiry-based laboratory experience, instructional activities, and AP® Exam questions. A Learning Objective merges required content with one or more of the seven Science Practices.
| Big Idea 4 | Biological systems interact, and these systems and their interactions possess complex properties. |
| Enduring Understanding 4.A | Interactions within biological systems lead to complex properties. |
| Essential Knowledge | 4.A.1 The subcomponents of biological molecules and their sequence determine the properties of that molecule. |
| Science Practice | 7.1 The student can connect phenomena and models across spatial and temporal scales. |
| Learning Objective | 4.1 The student is able to refine representations and models to explain how the subcomponents of a biological polymer and their sequence determine the properties of that polymer. |
| Science Practice | 1.3 The student can refine representations and models of natural or man-made phenomena and systems in the domain. |
| Learning Objective | 4.2 The student is able to refine representations and models to explain how the subcomponents of a biological polymer and their sequence determine the properties of that polymer. |
| Science Practice | 6.1 The student can justify claims with evidence. |
| Science Practice | 6.4 The student can make claims and predictions about natural phenomena based on scientific theories and models. |
| Learning Objective | 4.3 The student is able to use models to predict and justify that changes in the subcomponents of a biological polymer affect the functionality of the molecules. |
The Science Practices Assessment Ancillary contains additional test questions for this section that will help you prepare for the AP® exam. These questions address the following standards:
- [APLO 4.15]
- [APLO 2.5]
Molecular Structures
Molecular Structures
Most people are familiar with carbohydrates, one type of macromolecule, especially when it comes to what we eat. To lose weight, some individuals adhere to low-carb diets. Athletes, in contrast, often carb-load before important competitions to ensure that they have enough energy to compete at a high level. Carbohydrates are, in fact, an essential part of our diet; grains, fruits, and vegetables are all natural sources of carbohydrates. Carbohydrates provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many staple foods. Carbohydrates also have other important functions in humans, animals, and plants.
Carbohydrates can be represented by the stoichiometric formula (CH2O)n, where n is the number of carbons in the molecule. In other words, the ratio of carbon to hydrogen to oxygen is 1:2:1 in carbohydrate molecules. This formula also explains the origin of the term carbohydrate: the components are carbon (carbo) and the components of water (hence, hydrate). Carbohydrates are classified into three subtypes: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
Monosaccharides (mono- = one; sacchar- = sweet) are simple sugars, the most common of which is glucose. In monosaccharides, the number of carbons usually ranges from three to seven. Most monosaccharide names end with the suffix -ose. If the sugar has an aldehyde group—the functional group with the structure R-CHO—it is known as an aldose. If it has a ketone group—the functional group with the structure RC(=O)R'—it is known as a ketose. Depending on the number of carbons in the sugar, they also may be known as trioses (three carbons), pentoses (five carbons), and/or hexoses (six carbons). See Figure 3.5 for an illustration of the monosaccharides.
The chemical formula for glucose is C6H12O6. In humans, glucose is an important source of energy. During cellular respiration, energy is released from glucose, and that energy is used to help make adenosine triphosphate (ATP). Plants synthesize glucose using carbon dioxide and water, and glucose in turn is used for energy requirements for the plant. Excess glucose is often stored as starch that is catabolized—the breakdown of larger molecules by cells—by humans and other animals that feed on plants.
Two other common monosaccharides are galactose, or milk sugar, which is part of lactose, and fructose, which is found in sucrose and fruit. Although glucose, galactose, and fructose all have the same chemical formula (C6H12O6), they differ structurally and chemically, and are known as isomers, because of the different arrangement of functional groups around the asymmetric carbon. All of these monosaccharides have more than one asymmetric carbon (Figure 3.6).
Visual Connection
Identify each sugar as an aldose or ketose.
- fructose
- galactose
- glucose
- Glucose and galactose are aldoses. Fructose is a ketose
- Glucose and fructose are aldoses. Galactose is a ketose.
- Galactose and fructose are ketoses. Glucose is an aldose.
- Glucose and fructose are ketoses. Galactose is an aldose.
Glucose, galactose, and fructose are isomeric monosaccharides, or hexoses, meaning they have the same chemical formula but have slightly different structures. Glucose and galactose are aldoses, and fructose is a ketose.
Monosaccharides can exist as a linear chain or as ring-shaped molecules; in aqueous solutions monosaccharides are usually found in ring forms (Figure 3.7). Glucose in a ring form can have two different arrangements of the hydroxyl group (OH) around the anomeric carbon—carbon 1 that becomes asymmetric in the process of ring formation. If the hydroxyl group is below carbon number 1 in the sugar, it is said to be in the alpha (α) position, and if it is above the plane, it is said to be in the beta (β) position.
Disaccharides
Disaccharides
Disaccharides (di- = two) form when two monosaccharides undergo a dehydration reaction, also known as a condensation reaction or dehydration synthesis. During this process, the hydroxyl group of one monosaccharide combines with the hydrogen of another monosaccharide, releasing a molecule of water and forming a covalent bond. A covalent bond formed between a carbohydrate molecule and another molecule—in this case, between two monosaccharides—is known as a glycosidic bond (Figure 3.8). Glycosidic bonds, also called glycosidic linkages, can be of the alpha or the beta type.
Common disaccharides include lactose, maltose, and sucrose (Figure 3.9). Lactose is a disaccharide consisting of the monomers glucose and galactose. It is found naturally in milk. Maltose, or malt sugar, is a disaccharide formed by a dehydration reaction between two glucose molecules. The most common disaccharide is sucrose, or table sugar, which is composed of the monomers glucose and fructose.
Polysaccharides
Polysaccharides
A long chain of monosaccharides linked by glycosidic bonds is known as a polysaccharide (poly- = many). The chain may be branched or unbranched, and it may contain different types of monosaccharides. The molecular weight may be 100,000 daltons or more depending on the number of monomers joined. Starch, glycogen, cellulose, and chitin are primary examples of polysaccharides.
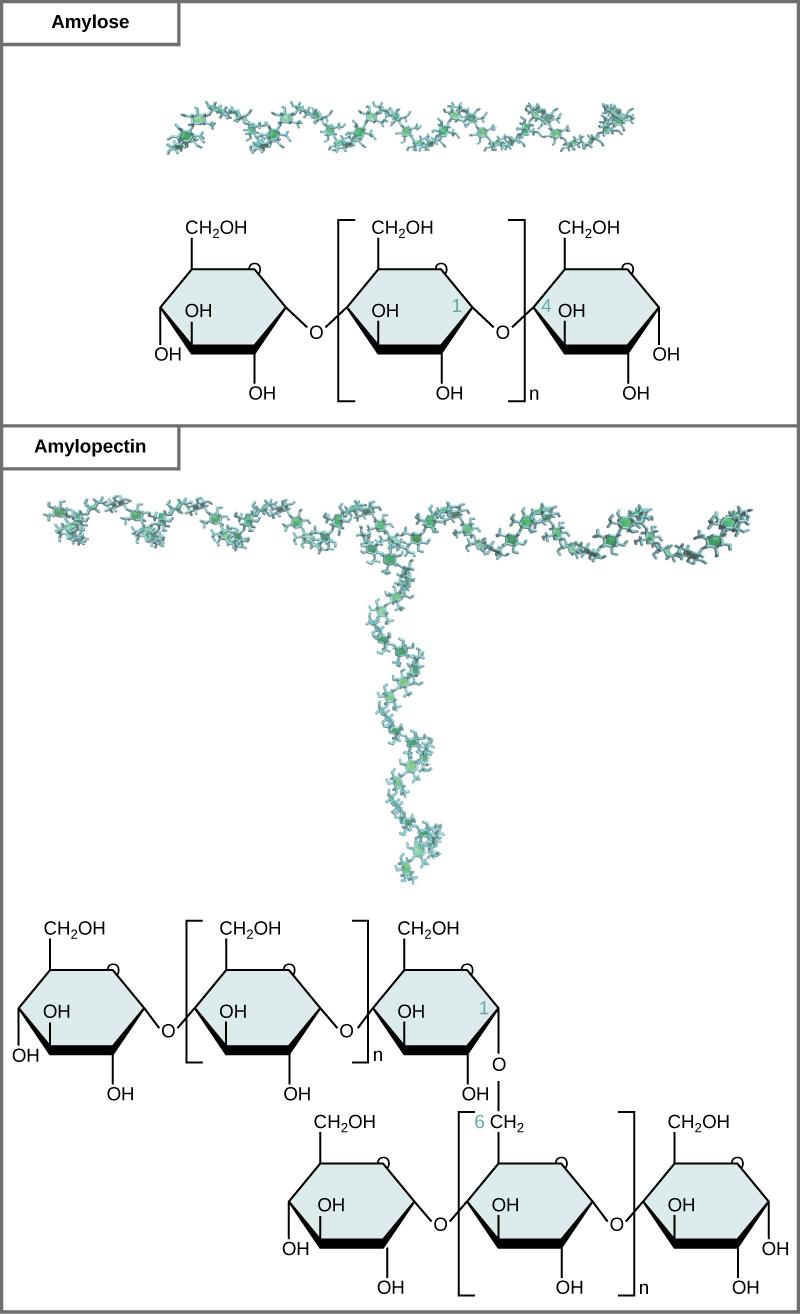
Starch is the stored form of sugars in plants and is made up of a mixture of amylose and amylopectin—both polymers of glucose. Plants are able to synthesize glucose, and the excess glucose beyond the plant’s immediate energy needs is stored as starch in different plant parts, including roots and seeds. The starch in the seeds provides food for the embryo as it germinates and can also act as a source of food for humans and animals. The starch that is consumed by humans is broken down by enzymes, such as salivary amylases, into smaller molecules, such as maltose and glucose. The cells can then absorb the glucose.
Starch is made up of glucose monomers that are joined by α 1-4 or α 1-6 glycosidic bonds. The numbers 1-4 and 1-6 refer to the carbon number of the two residues that have joined to form the bond. As illustrated in Figure 3.10, amylose is starch formed by unbranched chains of glucose monomers (only α 1-4 linkages), whereas amylopectin is a branched polysaccharide (α 1-6 linkages at the branch points).
Glycogen is the storage form of glucose in humans and other vertebrates, and is made up of monomers of glucose. Glycogen is the animal equivalent of starch and is a highly branched molecule usually stored in liver and muscle cells. Whenever blood glucose levels decrease, glycogen is broken down to release glucose in a process known as glycogenolysis.
Cellulose is the most abundant natural biopolymer. The cell wall of plants is mostly made of cellulose; cellulose provides structural support to the cell. Wood and paper are mostly cellulosic in nature. Cellulose is made up of glucose monomers that are linked by β 1-4 glycosidic bonds (Figure 3.11).
As shown in Figure 3.11, every other glucose monomer in cellulose is flipped over, and the monomers are packed tightly as extended long chains. This gives cellulose its rigidity and high tensile strength, which is so important to plant cells. While the β 1-4 linkage cannot be broken down by human digestive enzymes, herbivores such as cows, koalas, buffalo, and horses are able, with the help of the specialized flora in their stomach, to digest plant material that is rich in cellulose and use it as a food source. In these animals, certain species of bacteria and protists reside in the rumen, part of the digestive system of herbivores, and secrete the enzyme cellulase. The appendix of grazing animals also contains bacteria that digest cellulose, giving it an important role in the digestive systems of ruminants. Cellulases can break down cellulose into glucose monomers that can be used as an energy source by the animal. Termites are also able to break down cellulose because of the presence of other organisms in their bodies that secrete cellulases.
Carbohydrates serve various functions in different animals. Arthropods—insects, crustaceans, and others—have an outer skeleton, called the exoskeleton, which protects their internal body parts, as seen in Figure 3.12. This exoskeleton is made of the biological macromolecule chitin, which is a polysaccharide containing nitrogen. It is made of repeating units of N-acetyl-β-d-glucosamine, a modified sugar. Chitin is also a major component of fungal cell walls; fungi are neither animals nor plants and form a kingdom of their own in the domain Eukarya.
Career Connection
Registered dietitians help plan nutrition programs for individuals in various settings. They often work with patients in health care facilities, designing nutrition plans to treat and prevent diseases. For example, dietitians may teach a patient with diabetes how to manage blood sugar levels by eating the correct types and amounts of carbohydrates. Dietitians may also work in nursing homes, schools, and private practices.
To become a registered dietitian, one needs to earn at least a bachelor’s degree in dietetics, nutrition, food technology, or a related field. In addition, registered dietitians must complete a supervised internship program and pass a national exam. Those who pursue careers in dietetics take courses in nutrition, chemistry, biochemistry, biology, microbiology, and human physiology. Dietitians must become experts in the chemistry and physiology—biological functions—of food, namely proteins, carbohydrates, and fats.
Benefits of Carbohydrates
Benefits of Carbohydrates
Are carbohydrates good for you? People who wish to lose weight are often told that carbohydrates are bad for them and should be avoided. Some diets completely forbid carbohydrate consumption, claiming that a low-carbohydrate diet helps people to lose weight faster. However, carbohydrates have been an important part of the human diet for thousands of years; artifacts from ancient civilizations show the presence of wheat, rice, and corn in our ancestors’ storage areas.
Carbohydrates should be supplemented with proteins, vitamins, and fats to be parts of a well-balanced diet. Calorie-wise, a gram of carbohydrate provides 4.3 Kcal. For comparison, fats provide 9 Kcal/g, a less desirable ratio. Carbohydrates contain soluble and insoluble elements; the insoluble part is known as fiber, which is mostly cellulose. Fiber has many uses, including promoting regular bowel movements by adding bulk, and regulating the rate of consumption of blood glucose. Fiber also helps to remove excess cholesterol from the body—fiber binds to the cholesterol in the small intestine, then attaches to the cholesterol and prevents the cholesterol particles from entering the bloodstream, and then cholesterol exits the body via the feces. In addition, a meal containing whole grains and vegetables gives a feeling of fullness. As an immediate source of energy, glucose is broken down during the process of cellular respiration, which produces ATP, the energy currency of the cell. Without the consumption of carbohydrates, the availability of instant energy would be reduced. Eliminating carbohydrates from the diet is not the best way to lose weight. A low-calorie diet that is rich in whole grains, fruits, vegetables, and lean meat, together with plenty of exercise and plenty of water, is the more sensible way to lose weight.
Link to Learning
For an additional perspective on carbohydrates, explore Biomolecules: The Carbohydrates through this interactive animation.
Fiber is not really a nutrient because it passes through our body undigested. Which of the following statements best explains why fiber cannot be digested and why it is important to our diet.
- The enzymes required to digest cellulose are not produced in the human body; undigested fiber adds bulk to the food, easing bowel movements.
- The enzymes that digests cellulose cannot bind to the cellulose due to altered active sites; undigested fiber adds bulk to the food, easing bowel movements.
- The enzymes required to digest cellulose are not produced in the human body; fiber produces energy for the metabolism.
- Competitive inhibitors are not the reason that fiber is indigestible.
Science Practice Connection for AP® Courses
Activity
Use a molecular model kit to construct a polysaccharide from several different monosaccharide monomers. Explain how the structure of the polysaccharide determines its primary function as an energy storage molecule. Then use your model to describe how changes in structure result in changes in function.
Think About It
- Explain why athletes often carbo-load before a big game or tournament.
- Explain why it is difficult for some animals, including humans, to digest cellulose. Describe a structural difference between cellulose and starch, which is easily digested by humans. How are cows and other ruminants able to digest cellulose?
Disclaimer
This section may include links to websites that contain links to articles on unrelated topics. See the preface for more information.