Learning Objectives
Learning Objectives
By the end of this section, you will be able to do the following:
- Define nuclear fission
- Discuss how fission fuel reacts and describe what it produces
- Describe controlled and uncontrolled chain reactions
The information presented in this section supports the following AP® learning objectives and science practices:
- 1.C.4.1 The student is able to articulate the reasons that the theory of conservation of mass was replaced by the theory of conservation of mass-energy. (S.P. 6.3)
- 4.C.4.1 The student is able to apply mathematical routines to describe the relationship between mass and energy and apply this concept across domains of scale. (S.P. 2.2, 2.3, 7.2)
- 5.B.11.1 The student is able to apply conservation of mass and conservation of energy concepts to a natural phenomenon and use the equation to make a related calculation. (S.P. 2.2, 7.2)
- 5.G.1.1 The student is able to apply conservation of nucleon number and conservation of electric charge to make predictions about nuclear reactions and decays such as fission, fusion, alpha decay, beta decay, or gamma decay. (S.P. 6.4)
Nuclear fission is a reaction in which a nucleus is split (or fissured). Controlled fission is a reality, whereas controlled fusion is a hope for the future. Hundreds of nuclear fission power plants around the world attest to the fact that controlled fission is practical and, at least in the short term, economical, as seen in Figure 15.19. Whereas nuclear power was of little interest for decades following TMI and Chernobyl (and now Fukushima Daiichi), growing concerns over global warming has brought nuclear power back on the table as a viable energy alternative. By the end of 2009, there were 442 reactors operating in 30 countries, providing 15 percent of the world's electricity. France provides over 75 percent of its electricity with nuclear power, while the United States has 104 operating reactors providing 20 percent of its electricity. Australia and New Zealand have none. China is building nuclear power plants at the rate of one start every month.
Fission is the opposite of fusion and releases energy only when heavy nuclei are split. As noted in Fusion, energy is released if the products of a nuclear reaction have a greater binding energy per nucleon () than the parent nuclei. Figure 15.20 shows that is greater for medium-mass nuclei than heavy nuclei, implying that when a heavy nucleus is split, the products have less mass per nucleon, so that mass is destroyed and energy is released in the reaction. The amount of energy per fission reaction can be large, even by nuclear standards. The graph in Figure 15.20 shows to be about 7.6 MeV/nucleon for the heaviest nuclei about 240), while is about 8.6 MeV/nucleon for nuclei having about 120. Thus, if a heavy nucleus splits in half, then about 1 MeV per nucleon, or approximately 240 MeV per fission, is released. This is about 10 times the energy per fusion reaction, and about 100 times the energy of the average or decay.
Example 15.3 Calculating Energy Released by Fission
Calculate the energy released in the following spontaneous fission reaction,
given the atomic masses to be and
Strategy
As always, the energy released is equal to the mass destroyed times so we must find the difference in mass between the parent and the fission products.
Solution
The products have a total mass of
The mass lost is the mass of minus or
so the energy released is
Discussion
A number of important things arise in this example. The 171-MeV energy released is large, but a little less than the earlier estimated 240 MeV. This is because this fission reaction produces neutrons and does not split the nucleus into two equal parts. Fission of a given nuclide, such as does not always produce the same products. Fission is a statistical process in which an entire range of products are produced with various probabilities. Most fission produces neutrons, although the number varies with each fission. This is an extremely important aspect of fission, because neutrons can induce more fission, enabling self-sustaining chain reactions.
Spontaneous fission can occur, but this is usually not the most common decay mode for a given nuclide. For example, can spontaneously fission, but it decays mostly by emission. Neutron-induced fission is crucial as seen in Figure 15.20. Being chargeless, even low-energy neutrons can strike a nucleus and be absorbed once they feel the attractive nuclear force. Large nuclei are described by a liquid drop model with surface tension and oscillation modes, because the large number of nucleons act like atoms in a drop. The neutron is attracted and thus, deposits energy, causing the nucleus to deform as a liquid drop. If stretched enough, the nucleus narrows in the middle. The number of nucleons in contact and the strength of the nuclear force binding the nucleus together are reduced. Coulomb repulsion between the two ends then succeeds in fissioning the nucleus, which pops like a water drop into two large pieces and a few neutrons. Neutron-induced fission can be written as
where and are the two daughter nuclei, called fission fragments, and is the number of neutrons produced. Most often, the masses of the fission fragments are not the same. Most of the released energy goes into the kinetic energy of the fission fragments, with the remainder going into the neutrons and excited states of the fragments. Since neutrons can induce fission, a self-sustaining chain reaction is possible, provided more than one neutron is produced on average—that is, if in This can also be seen in Figure 15.21.
An example of a typical neutron-induced fission reaction is
Note that in this equation, the total charge remains the same (is conserved): Also, as far as whole numbers are concerned, the mass is constant. This is not true when we consider the masses out to 6 or 7 significant places, as in the previous example.
Not every neutron produced by fission induces fission. Some neutrons escape the fissionable material, while others interact with a nucleus without making it fission. We can enhance the number of fissions produced by neutrons by having a large amount of fissionable material. The minimum amount necessary for self-sustained fission of a given nuclide is called its critical mass. Some nuclides, such as produce more neutrons per fission than others, such as Additionally, some nuclides are easier to make fission than others. In particular, and are easier to fission than the much more abundant Both factors affect critical mass, which is smallest for
The reason and are easier to fission than is that the nuclear force is more attractive for an even number of neutrons in a nucleus than for an odd number. Consider that has 143 neutrons, and has 145 neutrons, whereas has 146. When a neutron encounters a nucleus with an odd number of neutrons, the nuclear force is more attractive, because the additional neutron will make the number even. About 2-MeV more energy is deposited in the resulting nucleus than would be the case if the number of neutrons was already even. This extra energy produces greater deformation, making fission more likely. Thus, and are superior fission fuels. The isotope is only 0.72 percent of natural uranium, while is 99.27 percent, and does not exist in nature. Australia has the largest deposits of uranium in the world, standing at 28 percent of the total. This is followed by Kazakhstan and Canada. The US has only 3 percent of global reserves.
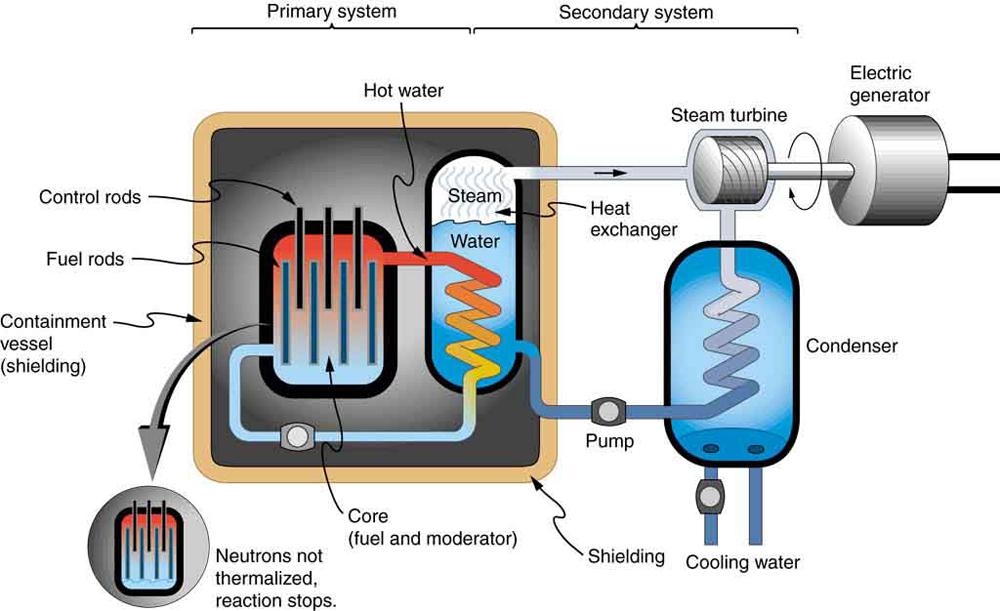
Most fission reactors utilize which is separated from at some expense. This is called enrichment. The most common separation method is gaseous diffusion of uranium hexafluoride through membranes. Since has less mass than its molecules have higher average velocity at the same temperature and diffuse faster. Another interesting characteristic of is that it preferentially absorbs very slow moving neutrons (with energies a fraction of an eV), whereas fission reactions produce fast neutrons with energies in the order of an MeV. To make a self-sustained fission reactor with it is thus necessary to slow down (thermalize) the neutrons. Water is very effective, since neutrons collide with protons in water molecules and lose energy. Figure 15.22 shows a schematic of a reactor design, called the pressurized water reactor.
Control rods containing nuclides that very strongly absorb neutrons are used to adjust neutron flux. To produce large power, reactors contain hundreds to thousands of critical masses, and the chain reaction easily becomes self-sustaining, a condition called criticality. Neutron flux should be carefully regulated to avoid an exponential increase in fissions, a condition called supercriticality. Control rods help prevent overheating, perhaps even a meltdown or explosive disassembly. The water that is used to thermalize neutrons, necessary to get them to induce fission in and achieve criticality, provides a negative feedback for temperature increases. In case the reactor overheats and boils the water to steam or is breached, the absence of water kills the chain reaction. Considerable heat, however, can still be generated by the reactor's radioactive fission products. Other safety features, thus, need to be incorporated in the event of a loss of coolant accident, including auxiliary cooling water and pumps.
Example 15.4 Calculating Energy from a Kilogram of Fissionable Fuel
Calculate the amount of energy produced by the fission of 1.00 kg of given the average fission reaction of produces 200 MeV.
Strategy
The total energy produced is the number of atoms times the given energy per fission. We should therefore find the number of atoms in 1.00 kg.
Solution
The number of atoms in 1.00 kg is Avogadro's number times the number of moles. One mole of has a mass of 235.04 g; thus, there are The number of atoms is therefore,
So the total energy released is
Discussion
This is another impressively large amount of energy, equivalent to about 14,000 barrels of crude oil or 600,000 gallons of gasoline. But, it is only one-fourth the energy produced by the fusion of a kilogram mixture of deuterium and tritium as seen in Example 15.2. Even though each fission reaction yields about ten times the energy of a fusion reaction, the energy per kilogram of fission fuel is less, because there are far fewer moles per kilogram of the heavy nuclides. Fission fuel is also much more scarce than fusion fuel, and less than 1 percent of uranium is readily usable.
One nuclide already mentioned is which has a 24,120-y half-life and does not exist in nature. Plutonium-239 is manufactured from in reactors, and it provides an opportunity to utilize the other 99 percent of natural uranium as an energy source. The following reaction sequence, called breeding, produces Breeding begins with neutron capture by
Uranium-239 then decays
Neptunium-239 also decays
Plutonium-239 builds up in reactor fuel at a rate that depends on the probability of neutron capture by (all reactor fuel contains more than Reactors designed specifically to make plutonium are called breeder reactors. They seem to be inherently more hazardous than conventional reactors, but it remains unknown whether their hazards can be made economically acceptable. The four reactors at Chernobyl, including the one that was destroyed, were built to breed plutonium and produce electricity. These reactors had a design that was significantly different from the pressurized water reactor illustrated above.
Plutonium-239 has advantages over as a reactor fuel—it produces more neutrons per fission on average, and it is easier for a thermal neutron to cause it to fission. It is also chemically different from uranium, so it is inherently easier to separate from uranium ore. This means has a particularly small critical mass, an advantage for nuclear weapons.