Engage: Thermal Energy Smackdown
Which has the most thermal energy: An iceberg or a pot of boiling water?
[Note: The interactive activity below is best viewed with Internet Explorer 9, Chrome 29, or Mozilla Firefox 5.0 and higher.]
To retake the quiz, reload the page and then select "No" when the "Resume Quiz" dialog box appears.
Explore: Temperature and Energy
[Note: This simulation requires Java. For Windows users, run Sun Java 1.5.0_15 or later on XP/Vista/7. For Mac users, run Sun Java 1.5.0_15 or later on OS 10.5 or later.]
Follow the instructions to explore the simulation.
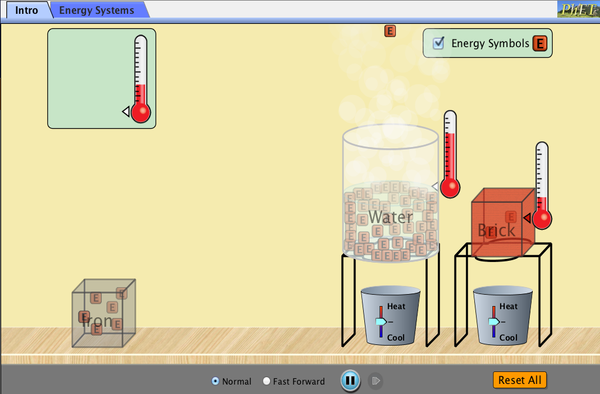
Part One: Set Up
- Click the image to run the simulation.
- When the simulation opens, check the box labeled “Energy Symbols.” The “E” symbol represents thermal energy.
- Place the thermometers onto the iron and brick blocks as shown. (Make sure the triangle next to the bulb of the thermometer is the same color as the object you are measuring.)
- Move the iron and brick blocks above the heat transfer devices.
Part Two: Exploration
- Use the lever on the heat transfer devices to make the amount of thermal energy in the brick and iron blocks equal. (Each “E” symbol = 1 thermal energy unit) How do the temperatures compare when the energies are equal? Record your observations in your science notebook.
- Use the lever on the heat transfer devices to make the temperatures of the brick and iron blocks equal. How does the amount of thermal energy compare when the temperatures are equal? Record your observations in your science notebook.
- Allow the brick and iron to reach the same temperature as the air.
- Place a thermometer in the water container. (Remember to check the triangle next to the bulb of the thermometer.)
- When all three substances reach the same temperature, compare the number of energy symbols in each. Why do you think that the amount of energy is unequal when the temperatures are equal? Record your answer in your science notebook.
Explain: Temperature and Energy
Temperature is related to, but not the same as, energy. Can you explain how they are similar and different? Watch the videos and record notes in your science notebook using a graphic organizer similar to the one shown.
Explain: Energy Concept Tree
Take a closer look at Nick Lucid’s energy concept tree from the Science Asylum's What Exactly Is Temperature? and What is Energy? videos. Looking at the concept tree, we can see why temperature is not the same as thermal energy.
Temperature is the average kinetic energy of the molecule in a substance whereas thermal energy is the total kinetic energy plus the potential energy of the molecules. The thermal energy in a particular substance depends on three things:
- Temperature of an object
- Amount of molecules in the substance (mass)
- Composition of the substance (including state of matter)
So, what about heat? Heat is just thermal energy on the move. Heat flows between substances of different temperatures. Objects have thermal energy, not heat. Objects do, however, transfer their thermal energy through heat. When heat is added to a system, the thermal energy of the molecules increases. When heat is released from a system, the thermal energy decreases. The temperature of the substance also increases or decreases accordingly unless the substance is undergoing a change in state. When substances change state, the potential energy between molecules changes, not the kinetic energy of the molecules.
Review your answer to this question from the thermal energy and temperature exploration: Why do you think that the amount of energy is unequal when the temperatures are equal? Make changes or additions to your answer to reflect your new learning. Compare your answer to the sample answer in the next section, Explain: Debrief
Explain: Debrief
Why do you think that the amount of energy is unequal when the temperatures are equal?
Sample Answer
The water has more energy than either the iron or brick because it is in the liquid state at room temperature. Particles in liquids have more kinetic energy than particles in solids. The water also has a larger volume than either of the two solid substances, so the total energy is greater even though the energy per molecule is the same.
The iron and brick are both solids and have the same volume, so the difference in total energy must be due to atomic composition. Different substances have different abilities to store energy.
Elaborate: Heating Curve Ball?
When you heat a substance from below its freezing point to above its boiling point, its temperature increases overall, but not steadily. Look at the graph below. This model heat curve shows the typical pattern: As thermal energy is added to any substance, the temperature increases then levels off, increases again, then levels off, and finally increases at the end. Does this seem strange to you? What’s going on?
Copy the generic heating curve into your science notebook. Using your knowledge of kinetic energy, potential energy, thermal energy, and temperature, label the following:
- Segments of the graph where the kinetic energy of the molecules is changing
- Segments of the graph where only the potential energy of the molecules is changing
Watch the video to check your answers.
Elaborate: Debrief
Kinetic energy (KE) changes when energy is gained or lost and the temperature changes.
Potential energy (PE) changes when energy is gained or lost and the temperature stays constant.
Evaluate: Concept Check
Check your understanding using the interactive quiz below.
[Note: The interactive activity below is best viewed with Internet Explorer 9, Chrome 29, or Mozilla Firefox 5.0 and higher.]
To retake the quiz, reload the page and then select "No" when the "Resume Quiz" dialog box appears.
Teacher Notes
This resource is a curated collection of interactives, videos, or other digital media assembled in a conceptually scaffolded 5E lesson format. It provides alternative or additional tier-one learning options for students learning about forms of energy, Chemistry TEKS (11)(A). The assignments require student participation with self-checked and teacher-checked formative assessment opportunities. For example, after students record observations and data in their notebooks, they may be prompted to be prepared to share their answers with the class.
Check for prerequisite knowledge, differentiation needs, and student follow-up requirements (as necessary) by reviewing the resource before assigning it to or working through it with your students.
Critical Vocabulary
- Thermal Energy
- Temperature
- Heat
- Kinetic Energy
- Potential Energy
Resource Map
How to Use this Resource
This resource can be used for instruction in a variety of ways.
- Use with a single computer and projector; this can be delivered in a traditional classroom.
- Use with a combination of individual student computers and teacher computer and projector (in either a computer lab or other 1:1 environment).
- Assign the resource to students as work to do outside of the school day as part of a flipped classroom to allow application, practice, and additional support during the school day.
- Use with students as tutorials.
- Share with parents to inform them about what their child is learning in school.
- Use with students who are unable to participate in the traditional classroom environment.